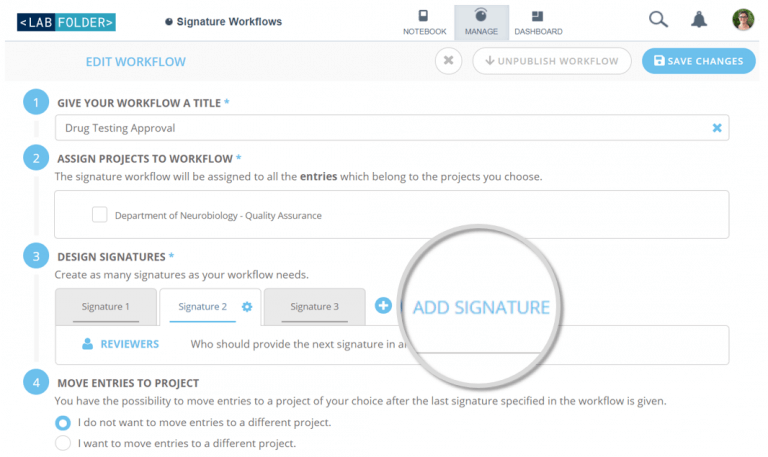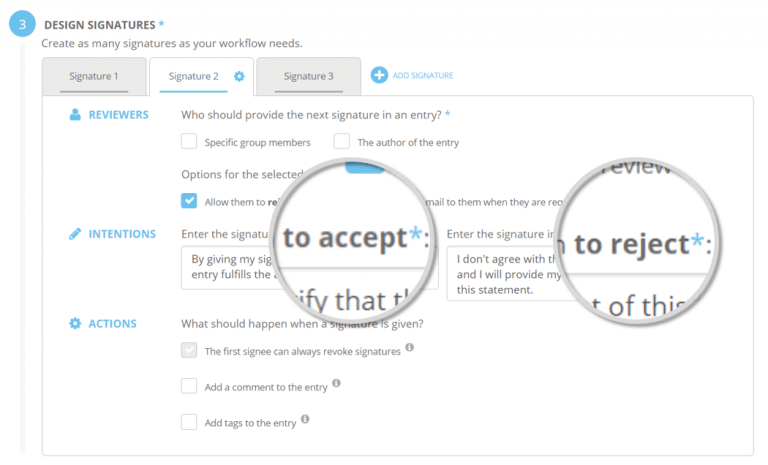
Signature Workflows enables compliant documentation and ensures quality of data through custom digital signatures. Create custom workflows for the reviewing process of your ELN entries in compliance with the FDA’s Title 21 CFR Part 11. An optional number of signatures can be designed, including intentions and actions to be applied upon approval or rejection. This optionally involves the re-location of specific entries, simplifying the processing by different departments.
For more information please visit our Signature workflows page.
Highlights: